Mask outlet
Time:2024-07-06 10:07
Source:Dongguan Global Electronic Technology Service Co.,Ltd.
Writer:Administrator
Can masks, protective clothing and other supplies be exported?
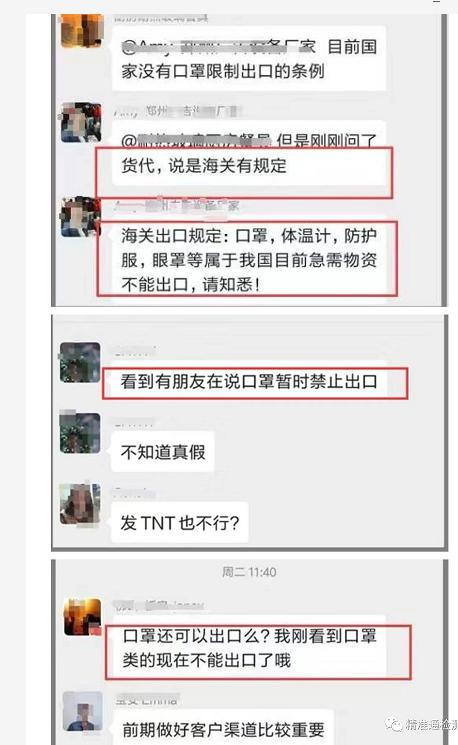
Recently, the global outbreak has led to a surge in demand for masks and other supplies. Some customers asked: Can masks, protective clothing, etc. be exported?
So we have specially researched and sorted out the policies on the export of masks and other epidemic prevention supplies and the actual situation of various countries. It is convenient for everyone to refer to and operate.
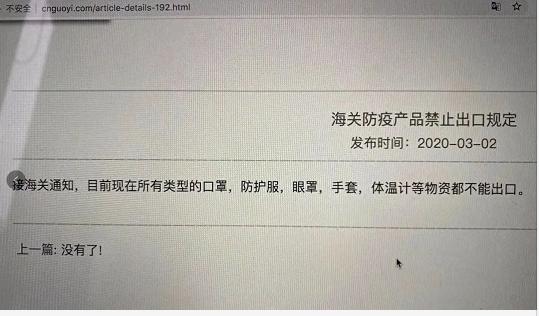
So is this notice circulating on the Internet true?
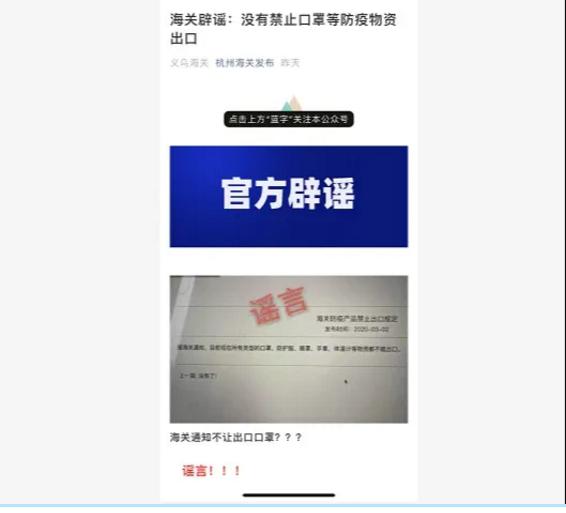
This is fake news!
Hangzhou Customs also officially refuted the rumor!
Ministry of Industry and Information Technology: Encourage the export of protective clothing overseas
Notice on the export of masks and other epidemic prevention materials
0 1 Reference for commodity codes of epidemic prevention materials
|
Urgently needed supplies
|
Reference tax number
|
|
Face mask
|
6307900000
|
|
Rubber gloves
|
4015190000
|
|
protective suit
|
6210103000
|
|
Goggles
|
9004909000
|
|
Cotton swabs, cotton balls
|
5601210000
|
|
thermometer
|
9025199090
|
|
disinfectant
|
3808940090
|
|
handwashing fluid
|
3401300000
|
02 What types of masks are there
? There are currently three types of masks in China:
the first type is masks managed as medical devices,
such as "medical protective masks", "disposable ordinary medical masks" and "medical surgical masks". Currently, medical masks are mainly these three types. To produce such products, it is necessary to apply for a "medical device product registration certificate" and a "medical device production license" from the device department of the provincial Food and Drug Administration. Currently, a clean workshop of 100,000 or above is required, and microbiological testing capabilities and related physical and chemical testing capabilities are required. The
second type is labor protection masks (special labor protection products)
. It is necessary to apply for an industrial product production license from the provincial Technical Supervision Bureau and apply for a "special labor protection product safety mark" certification ("LA" certification) from the State Administration of Work Safety.
When declaring the export of the above two types, you may be required to provide a copy of the production license or a foreign import registration form. Anyway, if you can produce it, these licenses are not a problem.
The third type is daily protective masks,
which are relatively simple. You do not need to apply for any licenses. You can send the product to a qualified third-party testing agency [Jingzhuntong Testing and Certification (Guangdong) Co., Ltd.] for inspection according to the corresponding standards, obtain the national authoritative test report, and have the CMA and CNAS accreditation marks, and then it can be put on the market for sale.
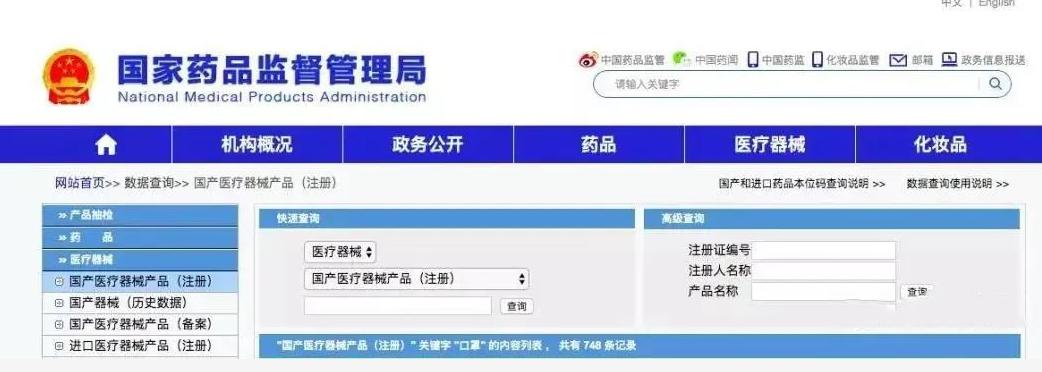
03 How to check whether the mask is qualified?
Log in to the official website of the "National Medical Products Administration" (www.nmpa.gov.cn), and then click on the "Medical Devices" - "Domestic Devices" column. According to the instructions on the page, enter the medical device registration certificate number or company name of the mask, etc., and you can know the company production information and approval number information related to the product, and you can know whether it is qualified.
04 Exports from China (company behavior)
for sale
require a medical device business license and import and export rights within the business scope before they can be exported.
For gifts or purchases
on behalf of others, or for purchases on behalf of affiliated companies (sister companies, parent and subsidiary companies), relevant qualification certificates of the purchasing manufacturer or the company's domestic manufacturer must be provided, which is the same as when we import, we need to provide three certificates abroad (business license, product medical device registration certificate, manufacturer inspection report).
05 What qualifications are required for masks exported to overseas countries?
1.
Necessary information (qualifications) in South Korea: bill of lading, packing list, invoice.
Business license of South Korean importers, South Korean consignees need to go to the Korea Pharmaceutical Traders Association. Korea to register import qualifications in advance (no, no), website: www.kpta.or.kr
If the company uses it for its own use and receives it as a gift, it can import it by itself without relevant qualifications.
Mask requirements:
Masks also need to have detailed origin identification. If they are made in China, they must be labeled: Made in China, manufacturer information, shelf life, and ingredient content descriptions and manufacturing process flow. These documents are not complete yet. The goods need to be monitored and tested after arriving in South Korea and samples must be sent to the laboratory. Only after the test is qualified can they enter the Korean market for sales and circulation.
2.
Necessary information (qualifications) in Japan: bill of lading, packing list, invoice.
PMDA-registered medical device companies exporting to Japan hope to put their products on the Japanese market. They must meet Japan's Pharmaceutical and Medical Device Act (PMD Act). Under the requirements of PMD Act, the TOROKU registration system requires foreign manufacturers to register manufacturer information with PMDA.
Mask requirements:
The words "Weirskate 99%" printed on the packaging are all medical masks that exceed the domestic filtration efficiency standard of 95% (N95 masks)!
PFE: 0.1um microparticle filtration efficiency
BFE: bacterial filtration rate
VFE: virus filtration rate
Weirskate: virus interception
1. Medical protective masks: in line with China's mandatory standard GB19083-2010, filtration efficiency ≥ 95% (tested using non-oily particles).
2. N95 masks: US NIOSH certification, non-oily particle filtration efficiency ≥ 95%.
3. KN95 masks: in line with China's mandatory standard GB2626, non-oily particle filtration efficiency ≥ 95%
3. EU
necessary information (qualification): bill of lading, packing list, invoice
Mask requirements:
In the EU, masks are classified as "substances and mixtures that endanger health." Starting from 2019, the new EU regulation PERegulation (EU) 2016/425 will be enforced. All masks exported to the EU must obtain CE certification under the requirements of the new regulations.
4.
Necessary information (qualifications) in the United States: bill of lading, packing list, invoice
Mask requirements:
Masks imported from the United States must be FDA certified before they can be sold in the domestic US market if they need to be sold. For masks for personal use and gifts, it is best to ask the US recipient whether FDA certification is also required when exporting, or purchase masks that have already passed FDA certification for export.
According to HHS (U.S. Department of Health and Human Services) regulations, NIOSH (National Institute for Occupational Safety and Health) divides its certified anti-particle masks into 9 categories. The specific certification is operated by the NPPTL laboratory under NIOSH. In the United States, masks can be divided into three levels according to the minimum filtration efficiency of the filter material-N, R, and P.
N-type masks can only filter non-oily particles, such as dust, acid mist, paint mist, microorganisms, etc. Most of the suspended particles in air pollution are also non-oily.
R masks are only suitable for filtering oily and non-oily particles, but the use time for oily particles is limited to no more than 8 hours.
P-type masks can filter both non-oily particles and oily particles. Oily particles include oil smoke and oil mist.
According to the different filtration efficiencies, there are 90, 95, and 100, which respectively refer to the minimum filtration efficiency of 90%, 95%, and 99.97% under the test conditions specified in the standard. N95 is not a specific product name. As long as it meets the N95 standard and passes the NIOSH review, it can be called an "N95 mask".
5. Necessary information (qualification) for Australia
: bill of lading, packing list, invoice
Mask requirements:
AS/NZS1716:2012 is the respiratory protection device standard for Australia and New Zealand, and the manufacturing process and testing of related products must comply with this specification. The standard specifies the procedures and materials that must be used in the manufacture of particle masks, as well as the testing and performance results that must be determined to ensure their safety in use.